The whole is greater than the sum of its parts, and, with chemical molecules, a combination of smaller atoms can make something greater, too.
Researchers in the lab of Chemistry Professor Amar Flood created a supramolecule that makes scientists re-think fundamental laws of chemistry and might have practical applications for fighting pollution.
“This was not planned at all,” said Elisabeth Fatila, postdoctoral researcher in Flood’s lab, on the surprise of making the discovery.
Fatila made the supramolecule, a molecule formed by weak interactions between other atoms and molecules.
“This discovery could provide new ways for us to more safely generate nuclear power by removing sulfate, which interferes with the storage of waste materials,” Flood said.
Though the molecule needs to be studied more thoroughly before seeing widespread commercial use, Flood said it could also provide ways to fight environmental pollution.
“We could also exploit the knowledge to make cheap and deployable sensors for phosphate in streams, rivers and lakes,” Flood said. “We imagine a future where citizen scientists could help monitor phosphate levels to alleviate environmental damage from over-fertilization and improve farming practices.”
This supramolecule would need some tinkering before use in nuclear waste systems because it is water-soluble, Fatila said, but the knowledge can help citizens develop useful molecules.
“We’ve gotten interest from people that deal with nuclear waste because that’s the over arching idea that they’re interested in,” Fatila said.
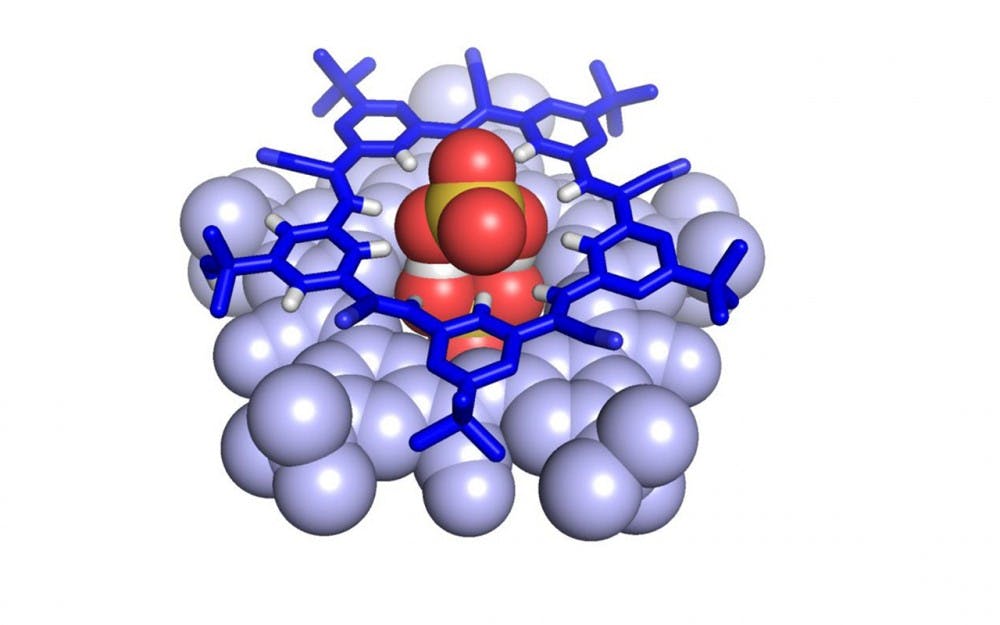
The supramolecule relied on negatively charged atoms, known as anions, of bisulfate.
They are normally unstable, but they stabilize one another when they come together.
“Having the anions come together makes the overall complex more stable,” Fatila said. “I didn’t realize how important that result was until Amar pointed it out to me.”
This stability allows the supramolecule to form with hydrogen bonds between two cyanostars, pentagonal molecules in five-sided star-shapes.
Fatima performed nuclear magnetic resonance, a process of detecting the resonant energy of the molecule in a magnetic field, to determine the molecule’s structure.
She also analyzed concentrations of the solution, the mass of the molecule and the light emitted by the solution.
Fatila could use these tests to ensure the molecule she was creating was the supramolecule.
This experimental discovery showed what theoretical data was suggesting, Fatila said.
The result is counterintuitive because two negatively charged atoms should normally repel, not bond with, one another, according to the scientific theory of Coulomb’s Law.
“It forces us to question our expectations of when matter does and does not follow the dictates of Coulomb’s law,” Flood said. “We always expect two anions to repel each other. Now that we have an example when they do not, we have to understand why.”
Fatila said she didn’t think the result was a big deal until she performed it and realized how unexpected it was.
“Big papers don’t come from ‘Oh, this totally explains my hypothesis,’ but usually ‘Oh, that’s odd,’” Fatila said.